What do inspectors find in ATMP production facilities?
Inspection findings
Numerous ATMP facilities have been inspected, resulting in several recurring issues (see Table 1). Many findings have led to new guidance and improved facility designs, enabling robust process designs and lowering contamination risks. Despite these improvements, inspection findings in ATMP facilities [continue to remain significant as of 2025].
From a regulatory perspective, upgrading a clinical trial drug product manufacturing site to commercial manufacturing can be a daunting task. For example, a facility design may be ill-suited for a commercial production campaign, the procedures may be too general or unspecific, and the staff may have the habit of prioritizing yields (a process development goal) over process control aspects of manufacturing.
Historically, many ATMP manufacturing sites emerge from multipurpose facilities such as university hospitals or clinical trial phase I production facilities. These facilities required a high degree of manufacturing flexibility, as they were designed for clinical supply, and thus introduced a degree of academic or sometimes hospital (exemption) rules into the cGMP playbook. The more established players – apart from having larger budgets – build facilities where the process defines the product and [tend to face] fewer facility risks, along with a reduced need to retrain staff to break ingrained habits.
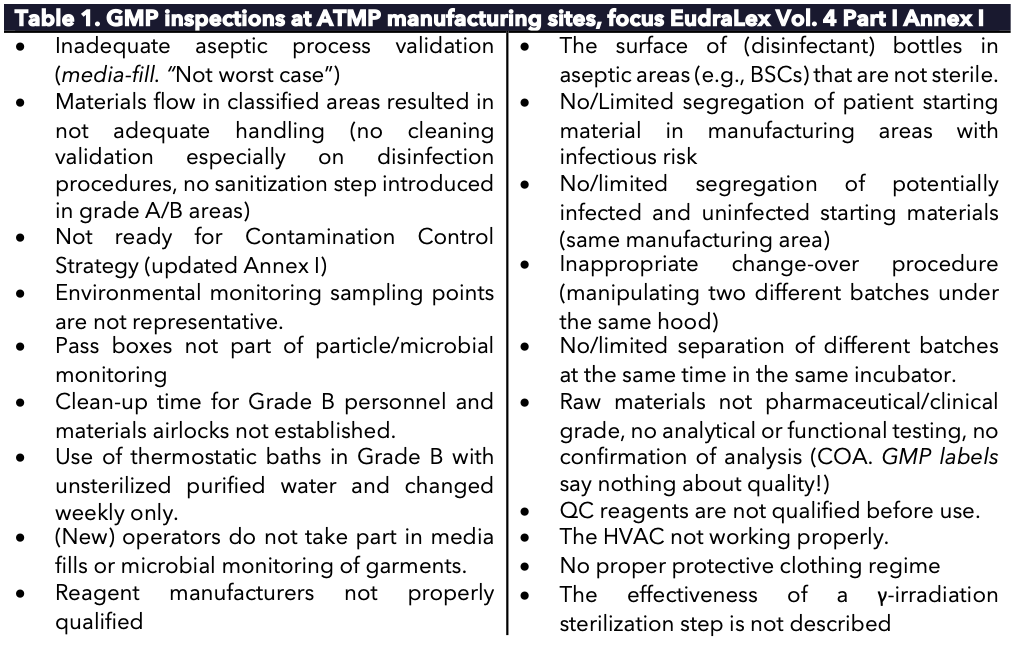
Table 1. GMP inspections at ATMP manufacturing sites, focus EudraLex Vol. 4 Part I Annex I.
Team up
On a positive note, [an increasing number of] nonprofit organizations (e.g., university hospitals) work closely together and support each other. For example, some organizations share extensive (and expensive) validation protocols and cleanroom designs so processes can be more easily transferred between centres and mistakes can be minimized.
What remains: manufacturing ATMPs is expensive, generally small-scale (USP-runs are typically <500 litres compared to antibody products easily reaching 10,000 litres), and frequently produced in multipurpose cleanrooms. This often results in a low number of batches produced across many different ATMP facilities, making it difficult to establish robust processes, retain experienced operators (due to high turnover rates), and prevent (viral) cross-contamination during line cleaning.
These challenges need to be carefully addressed, especially in light of the more elaborate process descriptions in [EU GMP Annex 1, which came into effect in 2023]. [The requirements are now considered industry-standard and should be fully integrated into operational procedures].
In line with this, the EU concept paper of 11 March 2025 (EMA/INS/GMP/48771/2025) proposes a revision of EudraLex Volume 4 Part IV – GMP Guidelines specific to ATMPs. The paper explicitly states that efforts are underway to integrate the “new” Annex 1 requirements into these ATMP-specific GMP guidelines. This revision is currently under stakeholder consultation, with formal adoption expected by March 2027. The harmonization aims to bridge regulatory expectations and practical implementation in complex ATMP manufacturing environments.
We also see an industry-wide push for automation in documentation, implementation of Pharma 4.0 principles, intensified (or continuous) production processes, and in-line live monitoring. [By 2025, these digital and automated approaches are no longer emerging trends but are increasingly expected in modern GMP environments]. The transition to these systems [remains] challenging for many ATMP facilities. [Reference frameworks such as ISPE GAMP 5 (Second Edition, 2022) and ISO 14644-4:2022 continue to offer essential guidance on system implementation and cleanroom design].
