Commissioning, Qualification and Validation
Who are we?
Progress has extensive experience in providing support throughout the entire product life cycle; from process development to end-of-life decommissioning. We have completed a broad portfolio of commissioning, qualification and validation (CQV) projects for clients producing small molecules, biologicals (proteins, Mabs, blood products), radiopharmaceuticals and ATMP (Cell and Gene therapy products). We take the product and its production process as the starting point for our qualification strategy for production processes, production or QC equipment, facilities and utilities. Our experts follow the current standards and guidelines, as described for example in the ISPE baseline 5 2nd edition Commissioning and Qualification and EU EudraLex – Volume 4 – Good Manufacturing Practice (GMP) guidelines.
Strategy
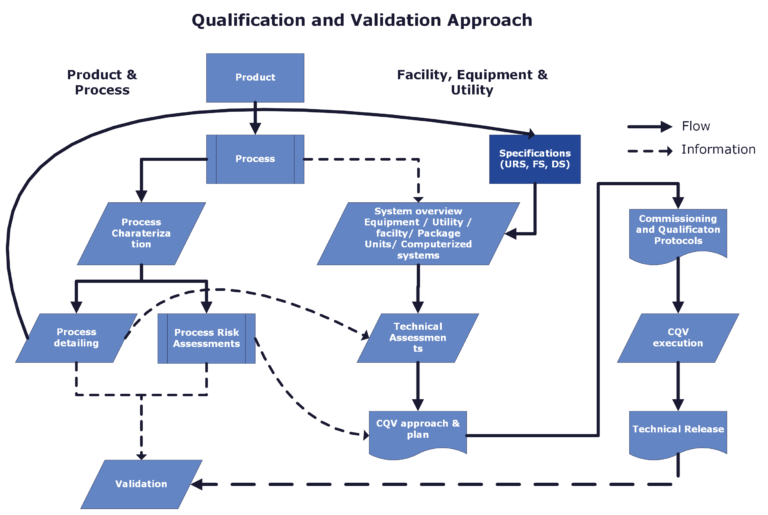
We have in-depth knowledge of (bio-)pharmaceutical processes, GMP and technical requirements for facilities, utilities and equipment. We use this knowledge to clearly define the commissioning, qualification and validation (CQV) strategy, in accordance with industry standards and guidelines. To meet client expectations with a compliant, value-added, and lean CQV process, we often use the following approach:
- The product is the starting point
- Define process details (CQA, CPP, process scale, number of process steps)
- Perform process risk assessments (Including – System Impact Assessments (SIA))
- Create specifications (Including URS)
- Create system/equipment overview
- Perform technical risk and impact assessments
- Define a commissioning, qualification and validation (CQV) approach
- Perform CQV program (design, execution, reporting)
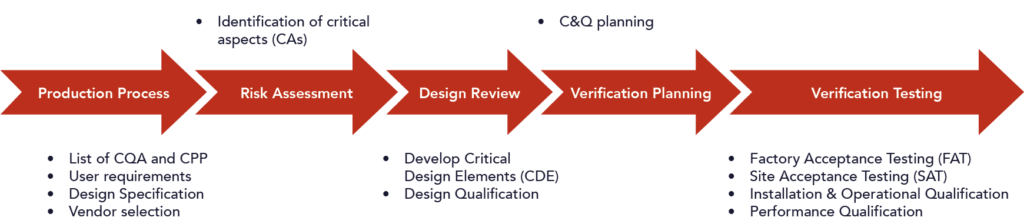
Planning and Implementation
Our consultants can assist you in the entire process of planning and executing all commissioning, qualification and validation activities. By using a risk-based approach, all possibilities, impossibilities, risks and time-critical action points are identified in advance, resulting in accurate planning. All risks will be closely monitored and timely action will be taken to prevent delays or mitigate risks.
Our experts can help you define the user requirements for your new facilities, utilities and equipment, and advise you on the selection and procurement process. A subsequent Design Qualification process ensures that the equipment design meets user requirements and specifications. Qualification protocols are then drawn up for the verification tests. During execution, we will be careful to perform the tests correctly and document the results according to good documentation practices.
Services
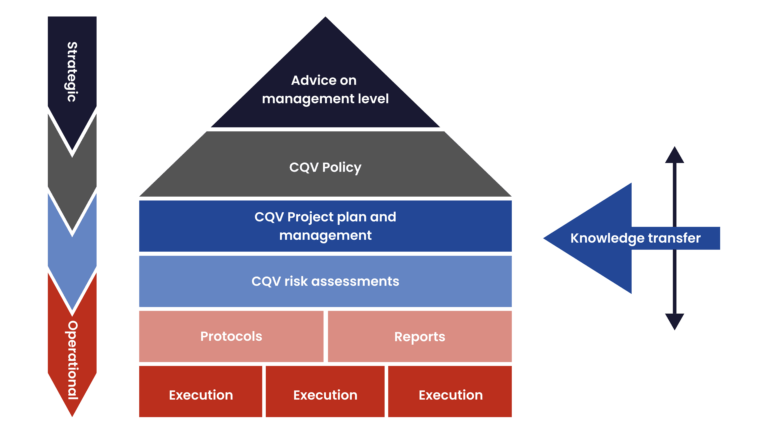
Progress offers the full range of services: from advice at management level to the implementation of protocols. At all levels we use risk-based qualification methods to accelerate and improve your qualification process, based on years of experience and the established ISPE baseline 5 2nd edition Commissioning and Qualification.
Progress meets the CQV needs for all areas of the pharmaceutical industry, including but not limited to OSD, sterile and aseptic manufacturing, biotechnology, ATMP and medical devices. Our employees are experts in CQV and guide your projects through the various phases according to planning and budget. They can support your organization, matching your needs from complete CQV teams or with one or more required roles, such as:
- Ad Interim Manager CQV department
- CQV Program or Project Manager
- CQV Planner / Coordinator
- CQV Engineer (e.g. CSV Engineer, Utility Engineer)
- Cleaning Validation (CV) Engineer
- CQV plans and protocols reviewer
We offer tailor-made services and specific support can be provided utilizing the extensive experience within the Progress team; for example, project management for technology transfer or support for operational and/or inspection readiness.
Track Record
Over the past 25 years we have made our mark in the field of commissioning, qualification and validation. Progress has actively helped many companies develop, implement and qualify new or modified facilities, utilities, equipment and systems. We take a science and risk-based approach, according to the latest regulations and standards.
Examples of successfully completed CQV projects include the commissioning and qualification of:
- cleanrooms (grade A-D) and related facilities
- utilities; such as purified water, WFI, compressed air, medical gases or waste water
- main equipment such as autoclaves (destruction, sterilization), washers, biosafety cabinets or LAFs, CIP systems
- a wide variety of production equipment; whether it is a bioreactor, lyophilizer, tablet press or packaging line or smaller equipment such as chromatography skids and UFDF systems.
- laboratory equipment; ranging from pH meters to LC-MS systems
- automated systems such as LIMS/ERP/MES/EMS
We are also experts in process validation, see Process Validation / PPQ – Progress (progress-lifesciences.nl), and cold chain validation.