Efficient and Timely Batch Review
Introduction
As managing Consultant and Quality Assurance specialist for Progress – Experts in Life Sciences I regularly perform audits on behalf of our customers at their suppliers and contract parties, or participate in internal inspections at the customer.
Case Description
Some time ago when I performed an audit at a pharmaceutical company a QA technician explained to me that they were hindered in their batch review work by the fact that the batch records were not offered to them until a long time (up to several months) after the manufacturing date.
As a consequence of the this long waiting period, the operators involved were often unable to recall specific situations in which recorded data was not sufficiently clear and raised questions for the QA technicians. In some cases, such situations led to deviation reports that could have been prevented.
Together we came to the conclusion that slow or postponed batch record review leads to different forms of waste with a significant risk of being unable to deliver in time, missed business opportunities, or even critical shortages in the market. Because of these risks we agreed to list this issue as an audit observation, classified as a “minor” observation.
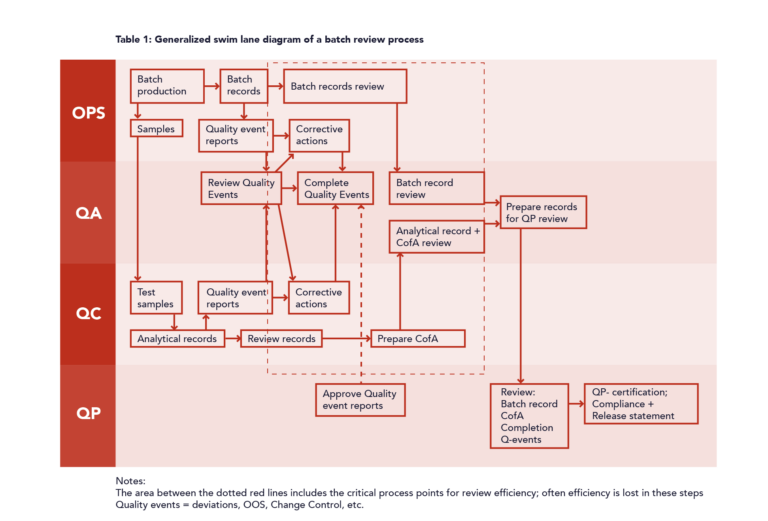
Moving Forward
After considering this issue in more depth I concluded that by applying a relatively simple set of instructions it would be possible to change the review process from a “push” to a “pull” principle and prevent delays and potential product shortages.
For all types of medicinal products, the manufacturer should do everything to prevent supply chain shortages, but for certain products with a short life span, or manufactured for a specific patient, it is especially important to complete a swift review to be able to release the product in time.
I proposed these 6 basic rules for establishing a timely review cycle:
- Make release an integral part of production.
The release testing and batch record review should be planned, and the planning adhered to. When planning a production run, also plan the steps beyond production until the final release of the batch. - Set acceptable throughput times.
There should be agreement on acceptable throughput times between operations, QC, QA and Supply Chain departments. Clearly define handover pre-requisites of batch documentation from executing party to reviewing party. The involved parties should work together and strive for an optimal process flow. - Prevent repetition of steps.
Try to prevent records going back and forth between QA and operations; prevent reprocessing in the review flow. For instance, by not sending documents for review with omissions that will most certainly have them returned (see also 2. “Define handover pre-requisites”) - Limit waiting times between steps.
Documents/ records should be completed at the time of operation, and there should be no storage or waiting. The review process should always be treated with priority. - Use process times as KPIs.
The acceptable throughput times (see point 3) should be set as KPIs. A KPI should be defined at least for the entire review process and preferably also for the throughput time of each individual process step . Department management should be responsible for monitoring and following up on these KPIs. - Escalate and celebrate.
Whenever the acceptable throughput times are exceeded, the issue should be escalated and actions defined to return to acceptable time frames. And, maybe even more importantly, celebrate each batch that is completed within the planned throughput time.
If all process steps are aligned, then completing the review should not take more time than what is needed to complete the QC release test requiring the longest time to complete (in most cases the sterility test).
Applying the above-listed rules and principles and striving for completion within the predefined time frame creates a pull mechanism in the release process, and such pull helps create predictable and plannable process times and prevents unnecessary waste of time.
Why Contact Progress - Experts in Life Sciences?
Our seasoned consultants have extensive knowledge of pharma, biotech and ATMP processes and are experienced in optimization processes (for instance using Lean techniques). They can analyze your review process, share best –practises, and make these fit perfectly for your situation to optimize your review process. This way you plan for success!
2. Set acceptable throughput times.
There should be agreement on acceptable throughput times between operations, QC, QA and Supply Chain departments. The involved parties should work together and strive for an optimal process flow.
3. Prevent repetition of steps.
Try to prevent records going back and forth between QA and operations by clearly defining handover pre-requisites of batch documentation from executing party to reviewing party. For instance, do not send documents for review with omissions that will most certainly have them returned.
