Mastering the Art of Cleaning Validation
Meeting the requirements for Cleaning Validation is complicated and indeed not a copy-paste task. It requires knowledge of law, guidelines, regulatory expectations, and awareness of the latest industrial developments. Every company, product, or production process is unique; that is why Cleaning Validation Life Cycle Management (CVLCM) is primarily a matter of customization.
Paul Tiemeijer, Cleaning Validation expert at Progress, explains: “Progress frequently receives requests from companies that have difficulty assessing whether their Cleaning Validation approach complies with regulations. Determining which validation approach is most suitable and effective for the customer requires expertise and a pragmatic approach. Companies’ Cleaning Validation policies and procedures often fail to meet required standards or result in highly inefficient validation programs. In some cases, this links to old mindsets and complex equipment-product portfolio configurations, which cause challenging cleaning validation matrix approaches. Regulatory expectations are also changing, for example, stricter rules about the Permitted Daily Exposure (PDE) within the EU or increasing focus on process capability from the FDA. Additionally, the growing emphasis on a risk-based approach to Cleaning Validation has changed how companies approach compliance, with regulators expecting a more systematic evaluation of cleaning risks and life cycle management.”
Achieving Compliance Without Compromising (OEE) Production Process Availability
Paul continues: “A successful Cleaning Validation approach requires not only proper procedures but also good practices, awareness, and a viable strategy. An efficient and effective Cleaning Validation program should not only ensure compliance but also be practical to execute within the tight production schedules. The goal is to achieve regulatory compliance while minimizing production loss (process down time) due to Cleaning Validation activities, including the associated Dirty Hold Time (DHT). By optimizing Cleaning Validation strategies, Progress has developed an approach with the right balance between meeting compliance requirements and maintaining production efficiency. Progress has years of experience in this field and is the market leader in developing effective and efficient validation programs.”
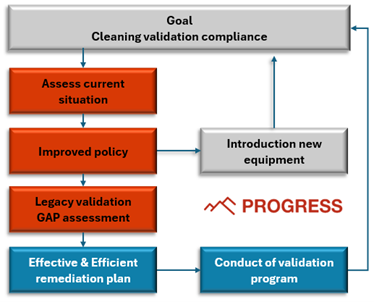
A Step-by-Step Approach to Smarter Cleaning Validation
Paul adds: “Based on a Quick Scan tool, Progress first assesses your current status. After this, we can, in consultation, optimize and adjust the policy and procedures. Based on the new procedures, a gap analysis is then carried out concerning new and old equipment or facilities. Ultimately, a mitigation plan is proposed. The added value we can provide is that every business has a different challenge, so a tailor-made approach is guaranteed, and the opportunity to coach and train people is always available.”
Ready to Optimize Your Cleaning Validation Approach?
At Progress, we are committed to providing tailored solutions that meet your specific needs and ensure regulatory compliance. Whether you need a program manager, support in execution, comprehensive gap analysis, customized training, or guidance on the latest regulations and technologies, our experts are here to help. Contact us today to schedule a consultation or learn more about our Cleaning Validation services and tailored training sessions.
